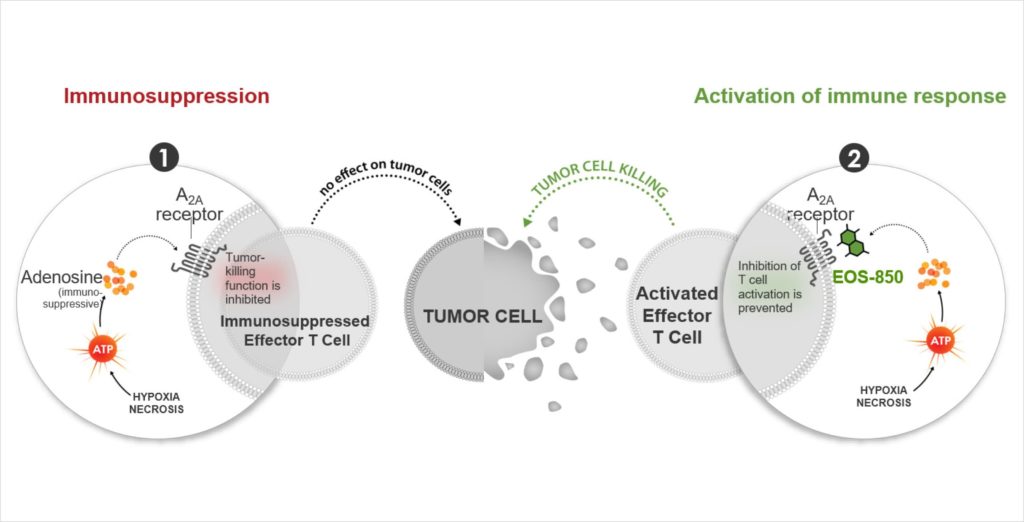
A treatment that targets immune cells’ primary adenosine receptor.
At iTeos we are pioneering the discovery and development of a new generation of highly differentiated immuno-oncology therapeutics.
EOS-448 is a human immunoglobulin antibody that we are developing to inhibit the immunosuppressive activity of TIGIT, a cell receptor expressed on multiple immune cells, including CD8+ T cells, natural killer (NK) cells, and T regulatory cells (Tregs).
Our differentiated development approach is driven by biologic rationale and clinical need utilizing our IO candidates in intra-portfolio combinations and with programs of external collaborators.
Latest press releases
iTeos Presents EOS-984 Preclinical Data Demonstrating Restoration of T Cell A…
WATERTOWN, Mass. and GOSSELIES, Belgium, April 07, 2024 (GLOBE NEWSWIRE) — iTeos Therapeutics, Inc. (Nasdaq: ITOS), a clinical-stage biopharmaceutical company…
iTeos Appoints Oncology Veteran Jill DeSimone to Its Board of Directors
WATERTOWN, Mass. and GOSSELIES, Belgium, March 06, 2024 (GLOBE NEWSWIRE) — iTeos Therapeutics, Inc. (Nasdaq: ITOS), a clinical-stage biopharmaceutical company…